12,000 Blood Treatments in China Found to be Contaminated With HIV
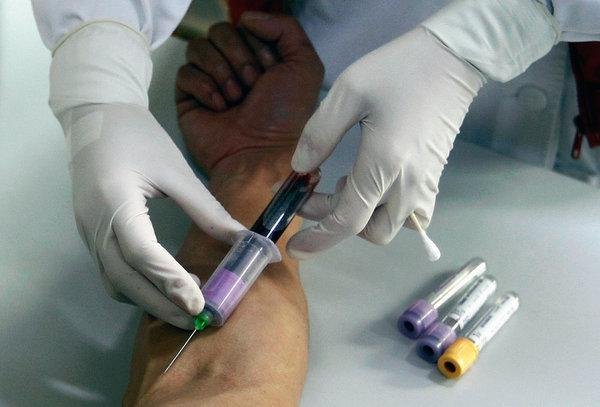
One of China’s largest state pharmaceutical companies found the HIV virus in 12,000 units of intravenous immunoglobulin, an immune therapy made with antibodies from blood plasma. The Shanghai Xinxing Pharmaceutical Company, China’s second-biggest medical blood products manufacturer, has notified China’s National Health Commission (NHC) and Shanghai Food and Drug Administration and the entire batch has been recalled.
The government has said it is possible those treated with the infected product could contract the disease and the company called for the government to “continuously observe and monitor patients who received the treatments.” The Jiangxi Provincial Disease Control center has not yet found anybody who has tested positive for HIV.
The NHC has warned Chinese hospitals to suspend the use of it after the disease control center in Jiangxi detected traces of HIV within it.
Failures in Chinese pharmaceutical manufacturing have forced the government to increase its monitoring of the sector.
Recently, more than 100 children were administered expired polio vaccines. Months earlier, faulty diphtheria, tetanus and whooping cough vaccines resulted in the government slapping a record penalty of an equivalent of 1.3 billion U.S. dollars on the vaccine manufacturer, Changchun Changsheng Biotechnology Company.
Last July, more than 250,000 contaminated rabies vaccines were produced by one of the country’s largest vaccine producers. The government ordered an inquiry, fined the company an equivalent of 1.7 million U.S. dollars and revoked the licence.



